Power Morcellation in Uterine Surgery
An Experienced Tampa Medical Device Attorney at Alley, Clark & Greiwe Can Help You Obtain Compensation Following a Power Morcellator Injury
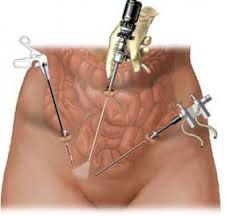 On November 24, 2014, the FDA issued an Updated Safety Communication addressed to the public, medical providers, hospitals, professional medical associations, and cancer advocacy groups warning against the use of medical devices called "power morcellators" commonly used in an estimated 50,000 laparoscopic surgeries per year to treat uterine fibroids.
On November 24, 2014, the FDA issued an Updated Safety Communication addressed to the public, medical providers, hospitals, professional medical associations, and cancer advocacy groups warning against the use of medical devices called "power morcellators" commonly used in an estimated 50,000 laparoscopic surgeries per year to treat uterine fibroids.
What is Morcellation?
Morcellation refers to the division of tissue into small pieces/fragments. A power morcellator has a fast rotating blade that breaks up the fibroid so that it can be removed via a small opening of a laparoscope.
New Power Morcellation Warnings
New studies indicate that approximately 1 in 350 women undergoing hysterectomy or myomectomy for the treatment of fibroids is found to have unsuspected uterine sarcoma - an aggressive, hard-to-detect type of cancer. Laparoscopic power morcellation poses a risk of spreading unsuspected cancerous tissue, notably uterine sarcomas, since morcellation can spray pieces of tumor beyond the uterus.
In April of 2014, the FDA warned against the use of power morcellation during hysterectomy or myomectomy given that there was no reliable method for predicting whether a woman with fibroids may have a uterine sarcoma. Health care providers and patients were strongly cautioned to consider available alternative treatment options for symptomatic uterine fibroids. The FDA issued a stronger warning against power morcellation on November 24, 2014.
Contact a Tampa Medical Device Attorney at Our Firm to Discuss Your Options in Filing a Power Morcellation Claim
If you or someone you know has been diagnosed with advanced uterine cancer after undergoing laparoscopic hysterectomy or myomectomy, please contact a Tampa defective medical device attorney Alley, Clark & Greiwe for information about your legal rights.




















