Breast Implant Cancer (BIA-ALCL)
Textured Breast Implants Linked to Rare Lymphoma
Since the introduction of breast implants in the 1960s, their safety has been debated extensively, even resulting in a temporary ban on silicone-gel implants for cosmetic indications by the FDA from 1992-2006. Today, breast augmentation is the most popular cosmetic surgical procedure in the U.S. and rates of breast augmentation and implant-based reconstruction are increasing each year.
FDA Warning in 2011
In 2011 the FDA announced a possible link between breast implants and a very rare form of non-Hodgkin’s lymphoma called anaplastic large-cell lymphoma (ALCL). The FDA warning reads, in part:
“Although ALCL is extremely rare, the FDA believes that women with breast implants may have a very small but increased risk of developing this disease in the scar capsule adjacent to the implant. Based on available information, it is not possible to confirm with statistical certainty that breast implants cause ALCL. At this time, data appear to indicate that the incidence of ALCL is very low, even in breast implant patients. Currently it is not possible to identify a type of implant (silicone versus saline) or a reason for implant (reconstruction versus aesthetic augmentation) associated with a smaller or greater risk.
The FDA is interested in learning more about the actual incidence of ALCL in women with breast implants, the characteristics of breast implants that might increase the risk of ALCL, and the pathological characteristics and clinical features of ALCL in women with breast implants. To this end, FDA is collaborating with the American Society of Plastic Surgeons to establish a registry of cases of women with breast implants who have been diagnosed with ALCL.”
Since the 2011 announcement, concerns about this rare lymphoma continue to grow.
What is known now about Breast Implant Associated Anaplastic Large-Cell Lymphoma?
Breast implant associated anaplastic large-cell lymphoma (BIA-ALCL) is a malignancy of the immune system. It is not breast cancer. BIA-ALCL usually arises in and around scar tissue and fluid near the breast implant. Based upon reportings of BIA-ALCL to date, what's inside the implant - silicone or saline - seems to make no difference. Nearly all cases of ALCL have been found with textured breast implants rather than implants with a smooth outer shell. What exactly causes the disease is unknown. One theory is that textured implants trap bacteria and form a coating that cases persistent inflammation and stirs up the immune system, which may eventually lead to lymphoma. Another theory is that the disease stems from an immune response to some component of the textured implant.
Most of the cancers found so far developed between 2-28 years after implant surgery, with a median of about 8 years. Researchers estimate that in Europe and the US one in 30,000 women with textured implants have a lifetime risk of developing BIA-ALCL. However, in Australia the estimate is higher: one in 1,000 to one in 10,000. No one knows why there is such a discrepancy, but it is clear that BIA-ALCL may have been under-reported and under-diagnosed for many years. Precise estimates are difficult to determine due to significant limitations in worldwide reporting and lack of global breast implant sales data.
Symptoms of the lymphoma usually include painful swelling and fluid buildup around the implant. Sometimes there are lumps in the breast or armpit. To make a diagnosis, doctors drain fluid from the breast and test it for a substance called CD30, which indicates lymphoma. When detected early, it can usually be cured by surgery alone by removing the implant and the capsule of scar tissue that forms around it. But some women need more extensive treatment with chemotherapy and radiation.
FDA Update About BIA-ALCL in 2017
In 2016 the World Health Organization added breast implant-associated ALCL (BIA-ALCL) as a new disease entity in revised lymphoma neoplasm classifications based on study data. The WHO described the usual presentation of the disease as an accumulation of seroma fluid between the implant itself and the surrounding fibrous capsule.
The FDA issued an update on BIA-ALCL in March of 2017. According to the update, the Agency said it had received 359 reports of BIA-ALCL which included 9 patient deaths. The FDA also said most confirmed cases of BIA-ALCL occurred in women with textured breast implants rather than smooth-surfaced implants regardless of filling type. By September 2017, the FDA reported the number of cases of BIA-ALCL had increased by 15% to 414 confirmed cases.
JAMA SURGERY Study in 2017 Says BIA-ALCL is Under-reported and Misunderstood
By late 2017 a review published in JAMA Surgery suggested BIA-ALCL was increasing in incidence and likely under-reported. The authors noted that there were significant limitations in worldwide reporting and a lack of a global breast implant database which made the exact number of cases of BIA-ALCL difficult to determine.
The researchers said almost all of the cases of BIA-ALCL were associated with textured implants, which have a slightly rough surface that keeps the implant in the correct position. Textured implants rose in popularity in the 1990s, and the first case of BIA-ALCL was documented in 1997. The researchers said that because they could find no incidents of BIA-ALCL prior to the introduction of textured implants, this suggested a causal relationship, although more research was needed.
After reviewing the literature, the researchers believed that BIA-ALCL occurred as a result of inflammation surrounding the breast implant and tissue that grows into the tiny holes in the textured implant may prolong that inflammation. Previous research has shown that chronic inflammation can lead to lymphoma.
Breast Implant Cancer Risk Highlighted in New 2018 JAMA Oncology Study
In January 2018 yet another study in the medical journal JAMA Oncology indicated textured breast implants appear to carry a 400-fold increased risk of breast anaplastic large-cell lymphoma (ALCL). While the overall risk of breast ALCL remained small, researchers indicated their findings almost guarantee a causal connection between breast implants and the cancer. Researchers looked at data from a nationwide Dutch pathology registry from 1990-2016 to identify all patients diagnosed with primary non-Hodgkin lymphoma of the breast. They looked at data on breast implant status, patient’s ages, and other types of breast lymphoma. According to the findings, 32 patients out of 43 with breast-ALCL had breast implants. Of the cases the researchers found, 82% involved macrotextured implants. Researchers concluded:
“Based on what is the largest population-based study conducted thus far, with nationwide coverage of breast-ALCL cases in the period from 1990 to 2016, we now confirm that implants strongly increase the risk of this rare type of lymphoma. Our relative risk estimate of over 400, implying an attributable risk approaching 100%, is highly suggestive of a direct or indirect causal role of the breast implant in breast implant-associated ALCL (BIA-ALCL).”
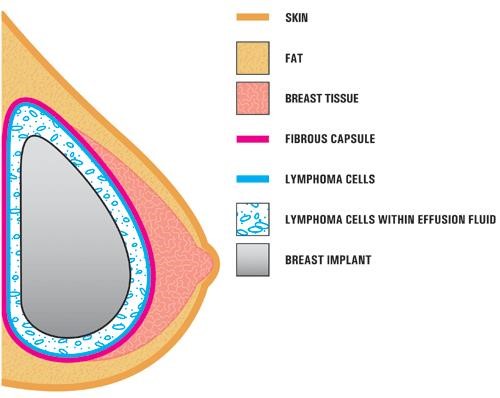
In the case studies reported in the literature, BIA-ALCL is usually found near the breast implant, contained within the fibrous scar capsule, and not in the breast tissue itself. (Photo courtesy of FDA)
The authors of the 2018 JAMA Oncology study offered three conclusions: 1.) comprehensive counseling of women considering breast implants should be mandatory including risks and symptoms of BIA-ALCL, 2.) alternative breast surgery procedures should be stimulated and most importantly, be reimbursed in specific groups of women, and 3.) calls for support of regulatory programs for breast implants and other medical devices, supported and funded independently as post-market monitoring systems.
Recommendations
If you are considering breast augmentation, you should talk to your physician about the benefits and risks of textured-surface vs. smooth-surfaced implants. If you already have breast implants, discuss appropriate routine surveillance with your physician.
Implant-associated ALCL is often very difficult to diagnose, so it is very important that you notify your doctor immediately if you have any unusual breast symptoms such as persistent swelling, painful swelling, lumps, or asymmetry.
Breast Implant Cancer Lawsuits
Based upon what is now know, information suggests that women with textured breast implants have a very low but increased risk of developing ALCL compared to women who do not have breast implants.
Alley, Clark & Greiwe continues to litigate a number of medical device cases. Each personal injury attorney at our law firm has extensive experience representing clients seriously injured due to defective medical devices and drug products. Our firm is reviewing potential textured breast implant lawsuits for women diagnosed with anaplastic large cell lymphoma (ALCL). If you have been diagnosed with non-Hodgkin's lymphoma of the breast, or ALCL from breast implants, please contact an experienced medical device attorney at Alley, Clark & Greiwe for a free consultation regarding your legal rights.
Consumer Links
Click here to read the study from JAMA Oncol. published online January 4, 2018
Click here to read the study from JAMA Surgery from October 2017
Click here to visit the FDA’s March 2017 update
Click here to visit the FDA’s January 2011 preliminary findings report
Click here to visit the FDA’s Q&A Page on BIA-ALCL
Click here to visit the WHO’s recognition of BIA-ALCL
Click here to visit the FDA’s breast implant website




















