News & Resources
November 26, 2014. Category: Product Liability, Unsafe Medical Devices
FDA Warns Against Use of Common Surgical Device Used to Treat Uterine Fibroids
On November 24, 2014, the FDA issued an Updated Safety Communication addressed to the public, medical providers, hospitals, professional medical associations, and cancer advocacy groups warning against the use of medical devices called "power morcellators" commonly used in an estimated 50,000 laparoscopic surgeries per year to treat uterine fibroids.
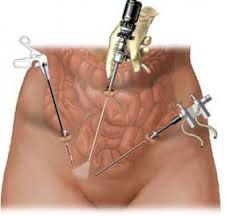
Click here to learn more about power morcellation and to read the FDA Safety bulletin from November 24, 2014.




















